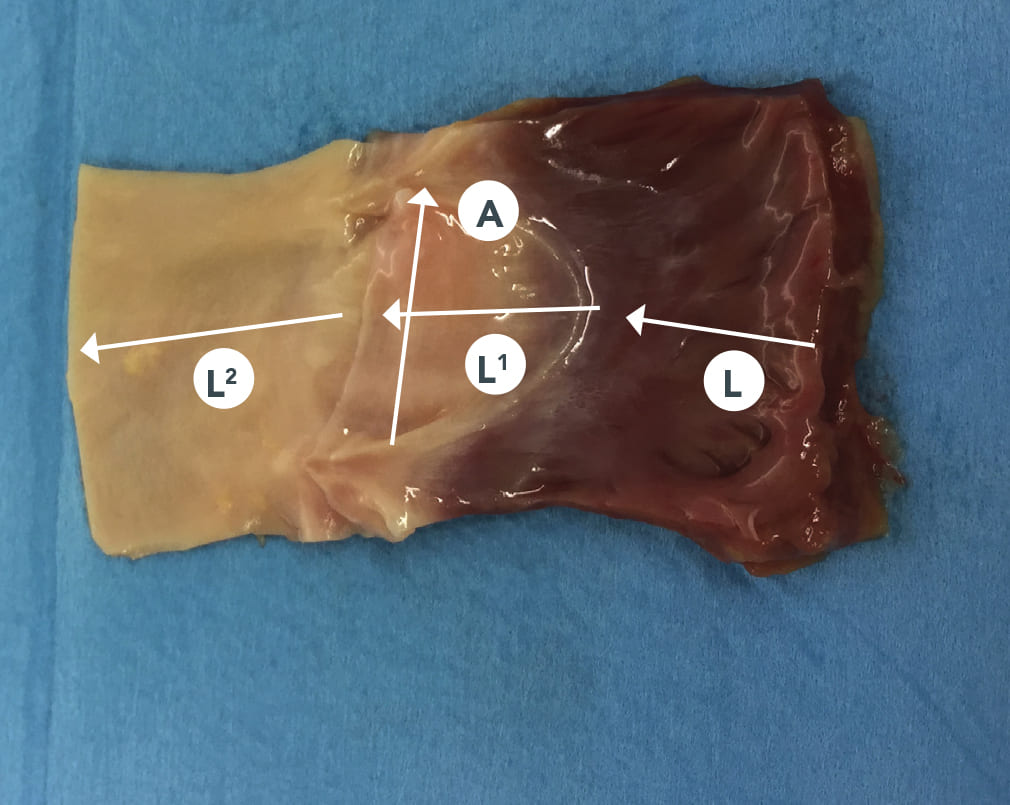
The pulmonary monocuspids supplied by FBTV consist of a single pulmonary valve flap with myocardium and arterial duct.
Used in cardiac surgery in the correction of congenital malformations such as Tetralogy of Fallot.
Homologous tissue comes from carefully selected donors, with a thorough evaluation of their medical and social history and thorough physical examination. Standard screening includes: Ac anti-HIV 1/HIV 2, Ac anti- HCV, HBsAg, Ac anti-HBc, Ac anti-HBs, Ac anti- Treponema pallidum, Ac anti-HTLVI/HTLVII, HBV-DNA, HCV-RNA, HIV- 1 RNA, Ac anti-CMV IgG and IgM. Microbiological screening and, if deemed necessary, additional tests are also performed on each donor and tissue. Tissues are harvested exclusively in the operating room to ensure sterility. The harvested tissue is kept at +2oC /+8oC until processing begins in FBTV’s classified laboratories.
Cardiovascular tissues are cryopreserved at -160°C, after immersion in a solution containing a cryoprotectant (DMSO), in order to minimise tissue damage due to water crystallisation during freezing, a process that is carried out by means of a computer-controlled temperature descent.
They are then stored in nitrogen vapour with continuous temperature monitoring.
The tissue is sent frozen in a container with dry ice, athermic, certified t o ensure temperature maintenance during transport. The shelf life of fabrics distributed and maintained at a temperature of -70°C/-80°C is 90 days from the date of shipment, one once
thawed must be used within 7 days. The Bank guarantees proper storage until receipt of the tissue; in the event of any problems occurring during transport, the user must immediately notify FBTV. All tissues distributed to of implantation are accompanied by documentation describing: donor suitability, origin and characteristics of the tissue, expiry date, serological and microbiological tests performed, method of preservation, instructions specific
related preservation and implantation, documentation for follow-up of the implanted tissue. Each tissue must be used for a single patient.
The results obtained to date show that homologous tissues, thanks to processing techniques, retain their original haemodynamic, inductive, conductive and elastic properties and can be considered first choice materials.
All preparation, handling and storage operations are carried out in an environment with facilities in compliance with national regulations. Laboratory activities are carried out in environments classified as Grade A according to the European Guide to Good Manufacturing Practices (GMP), the classification of the environments is verified with continuous checks in order to minimise contamination risks.
Access to classified rooms requires specific clothing to minimise possible contamination from outside and to safeguard the safety of personnel. Access to processing rooms is strictly limited to persons involved in the process or visitors accompanied by internal staff.
The processing of cardiac segments includes:
1. antibiotic decontamination with Gentamicin,
Vancomycin, Meropenem
2. packing the tissue in a double bag with cryoprotective
solution containing 10% DMSO
3. scheduled freezing and quarantine storage until all quality controls performed are reviewed and approved. Quality assurance and safety are performed:
A. a morphological evaluation of the fabric on arrival and during processing with grading according to a defined scale;
B. tissue decontamination by immersion in antibiotic solutions for 24-48h at +4°C;
C. culture tests for aerobic and anaerobic bacteria, fungi, during all activity phases.
The clinical use of human tissue offers important benefits for recipients. Like any product of human origin, their use is not without risks, although these are rare. A control system is needed to ensure traceability and identification of the tissue at any stage of the process, from donation to recipient. Each tissue is identified with a unique code to allow traceability from origin to destination. Once the tissue is transplanted the code must be attached to the recipient’s medical history. FBTV must be informed when the tissue has been transplanted, through the completion of the “Traceability Card” attached to the tissue. The bank must be informed if the tissue has not been implanted.
If there is suspicion or evidence of a serious adverse reaction or an effect in the recipient possibly related to the safety and quality of the transplanted tissue, the physician should contact immediately and complete the ‘Rapid Notification of Suspected Serious Adverse Events of Suspected Serious Adverse Events” or “Rapid Notification of Suspected Serious Adverse Reactions”.
FBTV is registered on the list of Tissue Banks accredited by the National Transplant Centre; regulated activities include donation, collection, evaluation, preparation, preservation and distribution of homologous tissues. It operates in compliance with international standard ISO 9001:2015 and has a certification process in place to certify that tissues meet predefined safety, traceability and morphological requirements. Homologous tissues procured, processed, stored and distributed meet the requirements of Italian legislation (Law 91/99, Legislative Decree 191/97, Legislative Decree 15/11/2016, Legislative Decree 256 16/12/2016, State/Regions Agreement 66/CSR 8/3/2018) and European Directive 2004/23 and Directives 2006/17/EC, 2006/86/EC, 2012/39/EU, 2015/565/EU, 2015/566/EU. FBTV follows the standards of the main scientific associations: European Association of Tissue Banks (EATB), American Association of Tissue Banks (AATB), and the recommendations of: Good Tissue Practices (Euro-GTP) and the Council of Europe EDQM Guide to the quality and safety of tissues and cells for human application.
Tutte le info per richiedere un tessuto incluso il modulo da compilare. Dopo aver ricevuto il vostro modulo, il nostro team procederà con una valutazione iniziale e vi contatterà.
oppure Accedi per scaricare il tariffario.
Fondazione Banca dei Tessuti del Veneto ETS / CF – P.IVA IT04478760269 / Privacy Policy